Happy Friday! It’s January 30th.
I’ve been building a small tool to track which AI-driven drug companies have programs in clinical trials. (Link below)
This week, I’m sharing the first public version of Company Trial Pulse. It links hand-vetted AI drug companies to trials in a U.S. clinical trial registry, so you can quickly see what’s really in Phase 1, 2, or beyond.
I’m starting with a curated Top 10 and a searchable view while I keep expanding and validating the data. Enjoy! And also, if you’ve got feedback or spot a bug, let me know!
Our picks for the week:
Feature: AI Parkinson Drug Ready For Phase 1 Trial
Perspective: Drug Trials Are Getting Faster, Not Smarter
Read Time: 4 minutes
FEATURED PERSPECTIVE
FDA Clears IND for Insilico ISM8969 As An AI-Developed Parkinson Drug Entering Phase 1 Testing

Insilico Medicine has been on a hot streak lately. Earlier this week, they announced that the FDA cleared its IND for ISM8969, an oral NLRP3 inhibitor aimed at Parkinson’s disease.
That clearance means the company can start a Phase 1 study in the U.S. to test safety, tolerability, and pharmacokinetics, basically how the body absorbs and processes the drug.
What they built: ISM8969 targets NLRP3, a protein complex linked to chronic inflammation. Insilico is betting that controlling this pathway could help protect neurons in neurodegenerative disease.
The company says the molecule is brain penetrant, which matters a lot when the target is in the central nervous system. Many therapeutics have a very difficult time passing the blood-brain barrier (as it should be).
The drug was discovered and optimized with Insilico’s generative chemistry platform, Chemistry42.
Insilico also notes the candidate was nominated in December 2024 as a potential best-in-class preclinical program for NLRP3, based on preclinical work it describes as showing favorable druggability and anti-inflammatory effects in mouse models.
The business angle: Following the greenlight by the FDA, Insilico also announced a co-development collaboration with Hygtia Therapeutics. The two parties will split global rights and interests 50-50, and Insilico is eligible to receive up to $66 million in upfront and milestone payments.
Granted, this is still very early; it’s only entering phase 1. Insilico just seems to be crushing it with back-to-back progress on these AI-developed drugs. Hopefully, we get to see other AI-drug companies follow in their path!
Sources: [Press Release]
Brain Booster
Which neurotransmitter is primarily deficient in the brains of individuals with Parkinson’s disease?
Select the right answer! (See explanation below and source)
What Caught My Eye
PHARMA AI
AI Won’t Replace Scientists, But It Will Replace Weeks of Work
Drugmakers are still waiting for the first clear “AI drug” (a drug designed completely with AI), but AI is already making a big impact on how clinical trials are run and how regulators are engaged.
Speaking this week at the JP Morgan Healthcare Conference, pharma executives described using AI to compress some of the slowest parts of the drug development cycle.
Clinical trials remain a major bottleneck. Bringing a drug to market can take more than a decade and roughly $2 billion. Companies say AI is now helping select trial sites, identify patients, and manage enrolment.
Novartis reported that AI reduced site selection for a 14,000-patient cardiovascular trial from several weeks to a two-hour meeting.
AI is also moving into regulatory work. Companies including AstraZeneca, Roche, and Pfizer are using AI to draft and cross-check thousands of pages of regulator-ready documents.
What stands out is where the progress is actually happening. AI is not changing how drugs work, but it is starting to take the drag out of everything around them. Fewer emails, fewer handoffs, fewer documents bouncing back and forth. Shaving time and cost from trial operations.
Source: [Full Article]
Byte-Sized Break
📢 Other Happenings in Healthcare AI
South Korea’s new AI law targets high-impact systems like medical diagnostics, requiring risk assessments and transparency, but faces criticism for vague definitions and limited protection for affected individuals. [Link]
Unauthorized “shadow AI” tools are widely used in U.S. hospitals, often without clinician guidance or governance, raising concerns about patient safety, data privacy, and the urgent need for enterprise-level AI policies in healthcare. [Link]
A study found Google’s AI Overviews cite YouTube more than any medical site for health queries, raising concerns about reliance on popularity over medical reliability, despite Google's claim that most top-cited videos come from trusted sources. [Link]
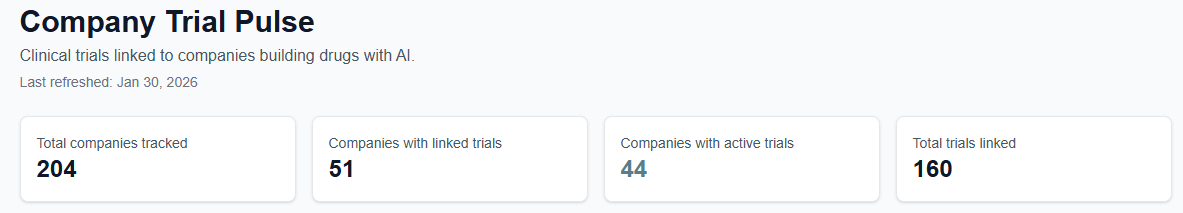
Clinical Trial Tracker
A few notes before you dive in. The companies here are manually reviewed, not scraped or auto-generated. Right now, trial data comes from a U.S. clinical trial registry only.
I’ve tested broader sources, but the data quality isn’t there yet, and accuracy matters more to me than coverage.
One more important caveat! The definition of “AI drug development” is still a moving target. Proving that AI is truly central to how a drug was developed is hard, and in many cases relies on trust, disclosures, and judgment calls rather than clean labels.
Expect this list to evolve as definitions sharpen and better evidence emerges.
Access the resource: [Link]
Have a Great Weekend!

❤️ Help us create something you'll love—tell us what matters!
💬 We read all of your replies, comments, and questions.
👉 See you all next week! - Bauris
Trivia Answer: A) Dopamine
Parkinson’s disease is a neurodegenerative disorder characterized by the loss of dopamine‑producing neurons in a part of the brain called the substantia nigra, which leads to a deficiency of the neurotransmitter dopamine and the movement problems seen in the disease. [Source]